Streamline your submissions and bring your drug to market with ease
We make FDA filings efficient and cost effective by enabling collaborative authoring and submissions in a modern, automated platform
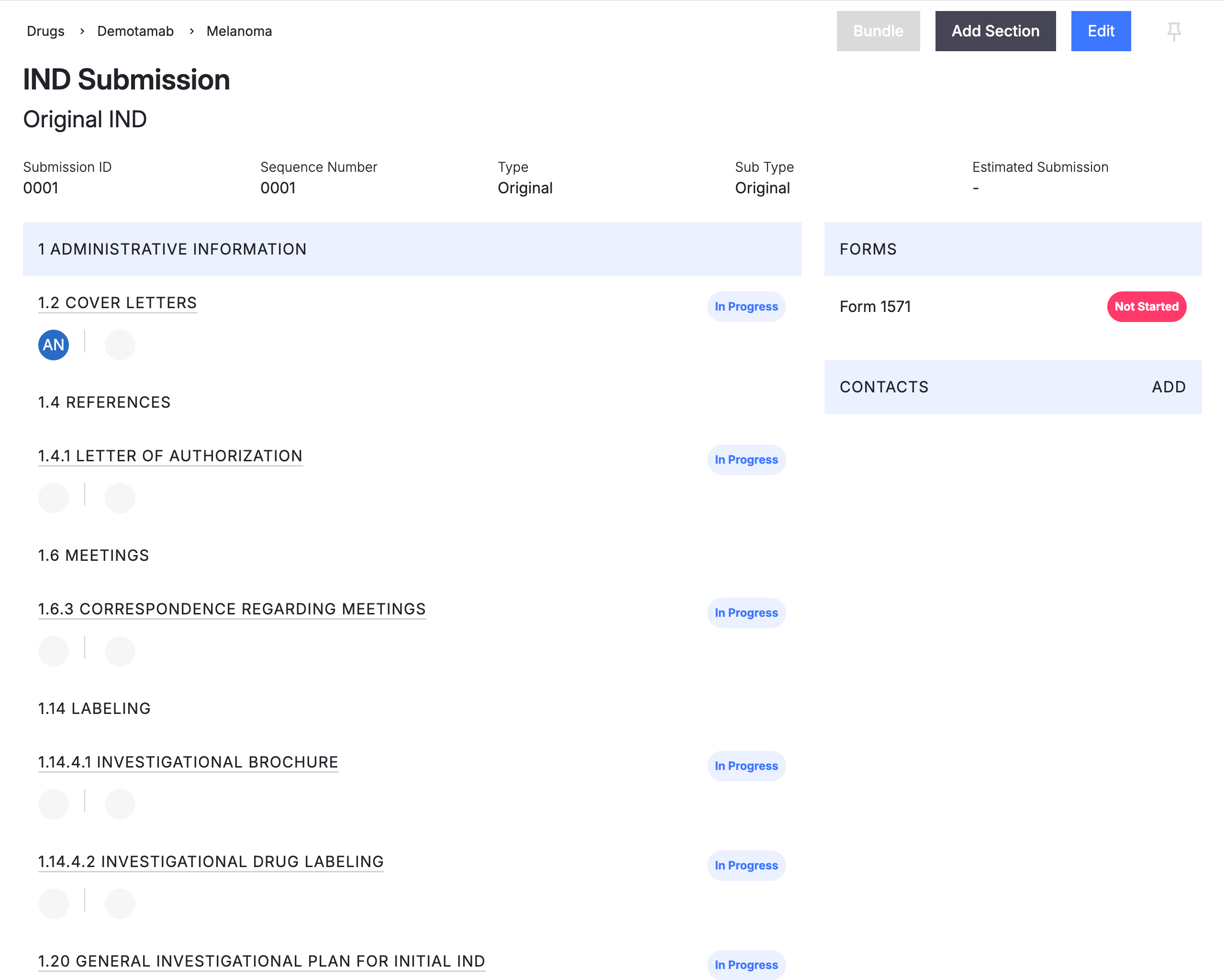
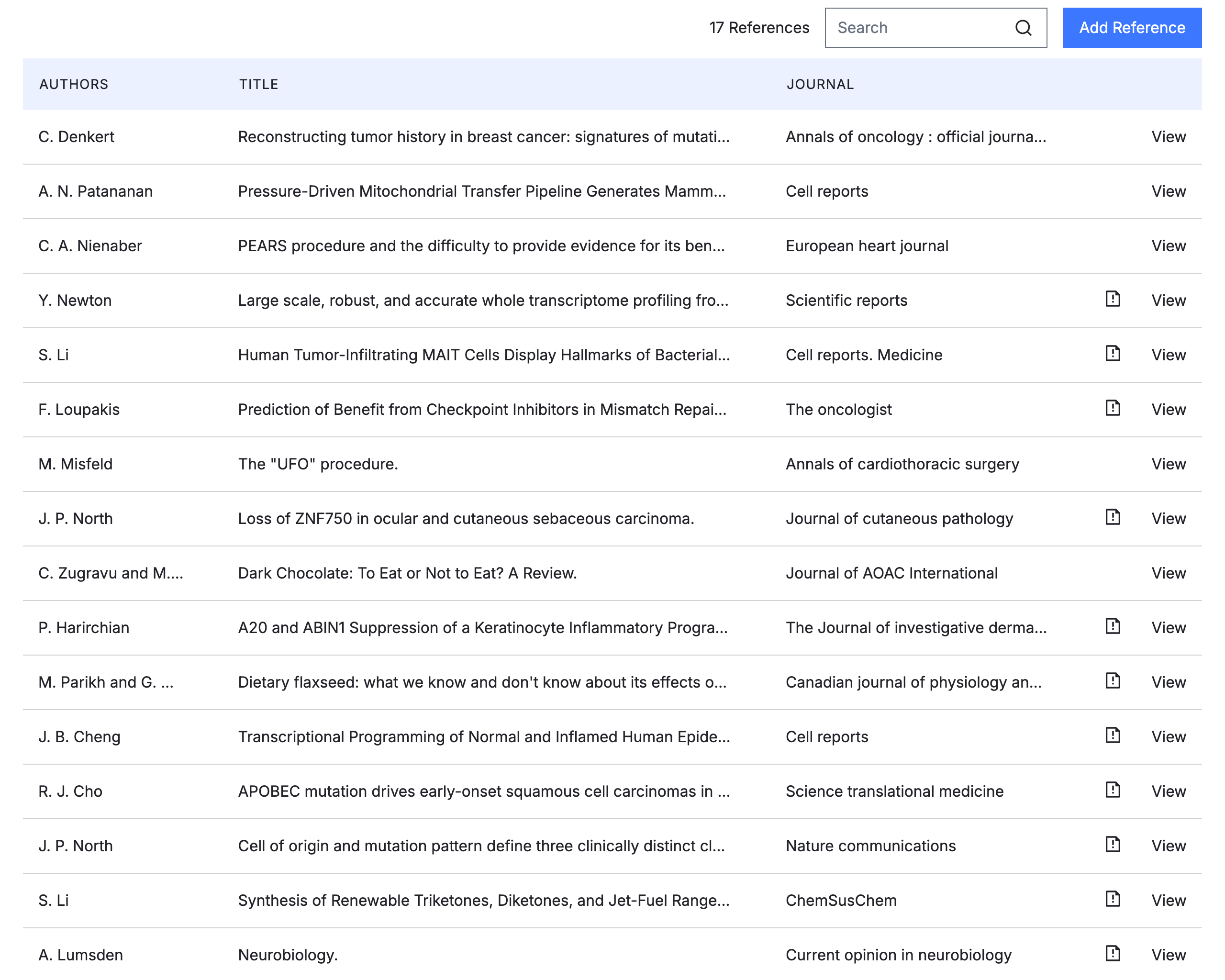
Regulatory Information Management (RIM) Simplified
Achieve full visibility on pending submissions from document authoring through completed sections while having easy access to past submissions
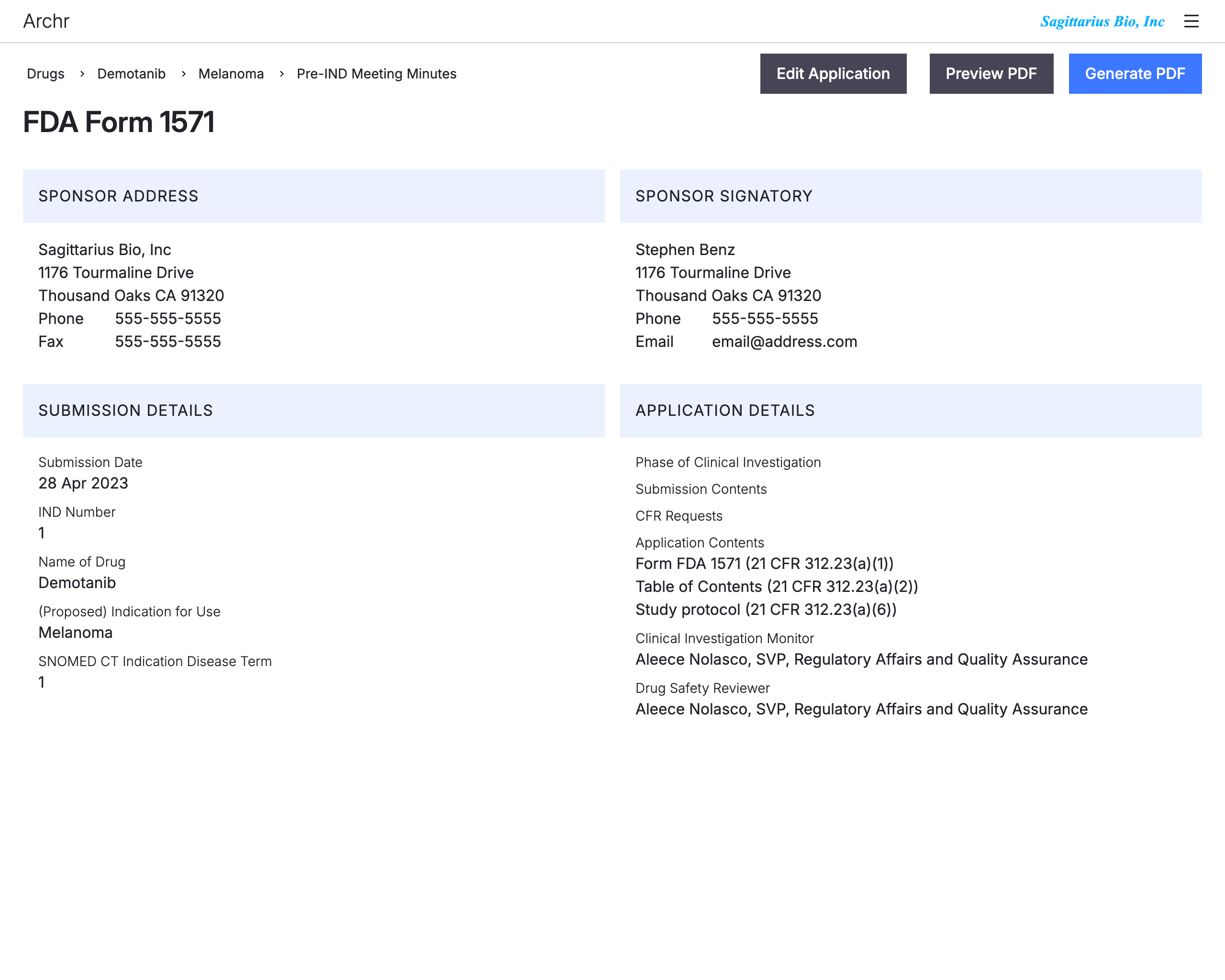
Assign sections
CTD Sections can be assigned to authors, subject matter experts and reviewers.
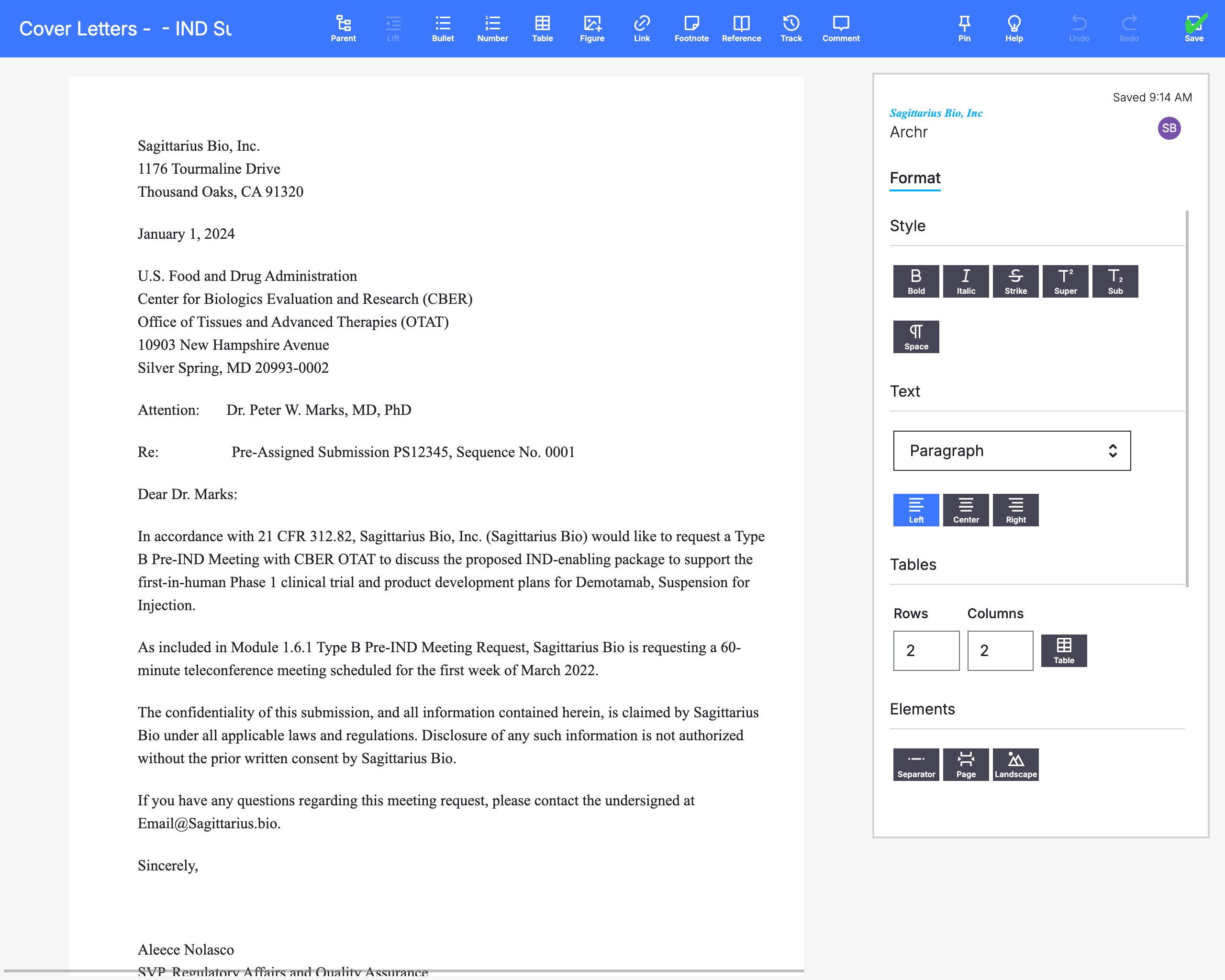
Populate Content
Previously submitted content can be intelligently recalled into a new submission to ensure consistency across documents.
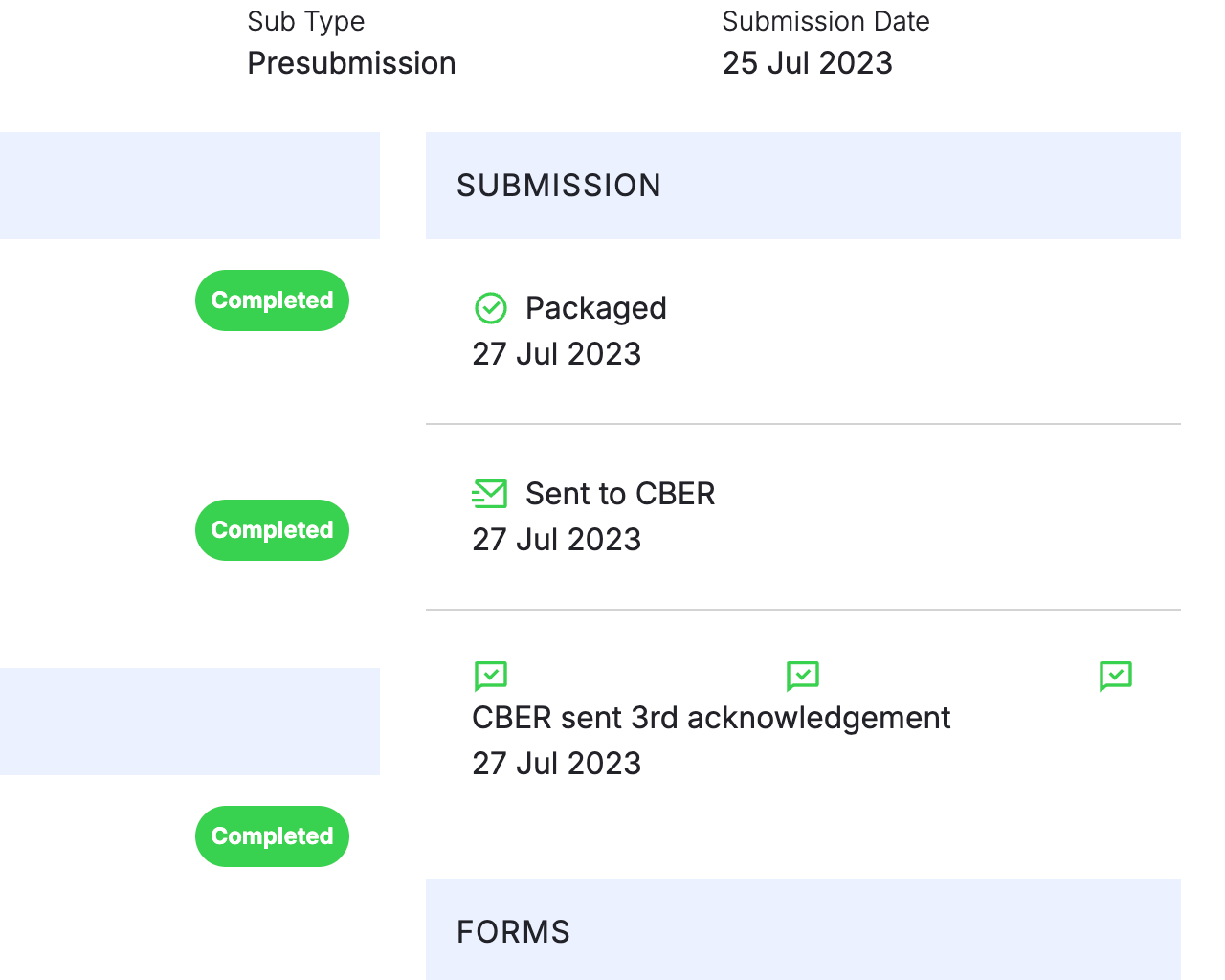
Track Correspondence and Submissions
Automatically populates a searchable log of your correspondence and submissions by application and indication for future reference.
Get Started
We offer straightforward pricing without charging implemention or per-seat annual license fees.
Contact Us for Pricing
Frequently Asked Questions
How quickly can I get going?
Archr is a web-based platform and you can begin authoring your next submission in CTD format today.Are there implementation fees?
There are no implementation fees for Archr. We offer straightforward pricing without charging per-seat annual license fees.Do you support international submissions?
Archr is currently focused on US FDA submissions. International submissions to EU, CAN, AUS are on the development roadmap. Please ask us for more information.Do I have to use the editor?
Archr's integrated editor is recommended due to the seamless authoring to submission experience, supporting intra- and inter-document linking and references. Existing authored documents in PDF format can also be easily uploaded to the platform for use in regulatory submissions. If you have questions about how this might fit with your existing authoring workflow, please reach out to us.How do I collaborate with users outside my organization (CROs, consultants, etc)?
Archr is a web-based platform that enables and encourages collaborative authoring and review of documents. Archr users outside your organization will be assigned individual login credentials. As a Sponsor, you can limit outside user access to review or full authoring access, by document or CTD Section.Do I need Regulatory Affairs (RA) experience to use Archr?
Specialized regulatory expertise is not needed to use Archr. In fact, Archr uses guided submission creation to help you determine the contents of your submission. Once your submission is final, it will generate bookmarked and hyperlinked PDFs that you can transmit to a regulatory agency.Do you integrate with pharmacovigilance, data management, quality and other industry-specific platforms?
Archr was designed to support third-party integrations. Please ask us for more information.Do I need Regulatory Operations (RegOps) experience to use Archr?
Specialized regulatory operations experience is not needed to use Archr. In fact, Archr will guide you step by step to bundle, validate and transmit your final submission to a regulatory agency.What infrastructure do I need to use Archr?
To use Archr, all you need is a modern web-browser. While smaller form factor devices will work, we recommend a desktop or laptop environment for the best authoring experience.Why does Archr leverage an AS2 connection to transmit submissions to a Regulatory Agency over the ESG?
Archr leverages the most recent technology to transmit submissions directly to a regulatory agency. Archr's direct AS2 connection eliminates the need to export your bundled submission to an external ESG software.I have yet to submit an IND or CTA to a Regulatory Agency, can I still use Archr?
It's never too early to use Archr. Archr is ideal and was designed for early development companies and their discovery and IND-enabling activities. Archr can be used to author and finalize study reports that will ultimately populate your future Investigational New Drug Applications (INDs).Does Archr comply with the specifications listed in the FDA eCTD Technical Conformance Guide?
Yes, Archr complies with the specifications listed in the FDA eCTD Technical Conformance Guide, Version 1.8, including version 3.2.2 of the ICH Electronic Common Technical Document Specification. ICH eCTD v4.0 support is currently on the development roadmap.Does Archr support Drugs, Biologics and Devices?
Drugs and biologics both can be authored and transmitted to a regulatory agency with Archr. Please ask us about device submissions.